Synairgen Plc and Southampton Clinical Trials Unit
Solving electronic consent for remote clinical trials
How do you conduct a clinical trial of non-hospitalised patients experiencing COVID-19 when those patients need to stay at home?
This was the problem facing Synairgen and Southampton Clinical Trials Unit in early 2020 at the start of the COVID-19 pandemic. Through Synairgen’s leading research they had identified a novel therapeutic drug called SNG001, which had previously been tested in patients suffering from COPD-related illnesses which cause breathing difficulties. SG016 is the name given to the trial that would test the drug in COVID-19 patients in a randomised double-blind clinical trial. The hope was that this drug would be able to help treat patients in a home setting and reduce hospitalisations.
To deliver this trial remotely, consent needed to be obtained from patients remotely. This was the problem that 10 Degrees helped solve by developing a regulatory-approved electronic consent platform for the clinical trials staff, nurses, doctors and patients to use.

Questions and problems we helped solve during the project
How feasible is an electronic consent process?
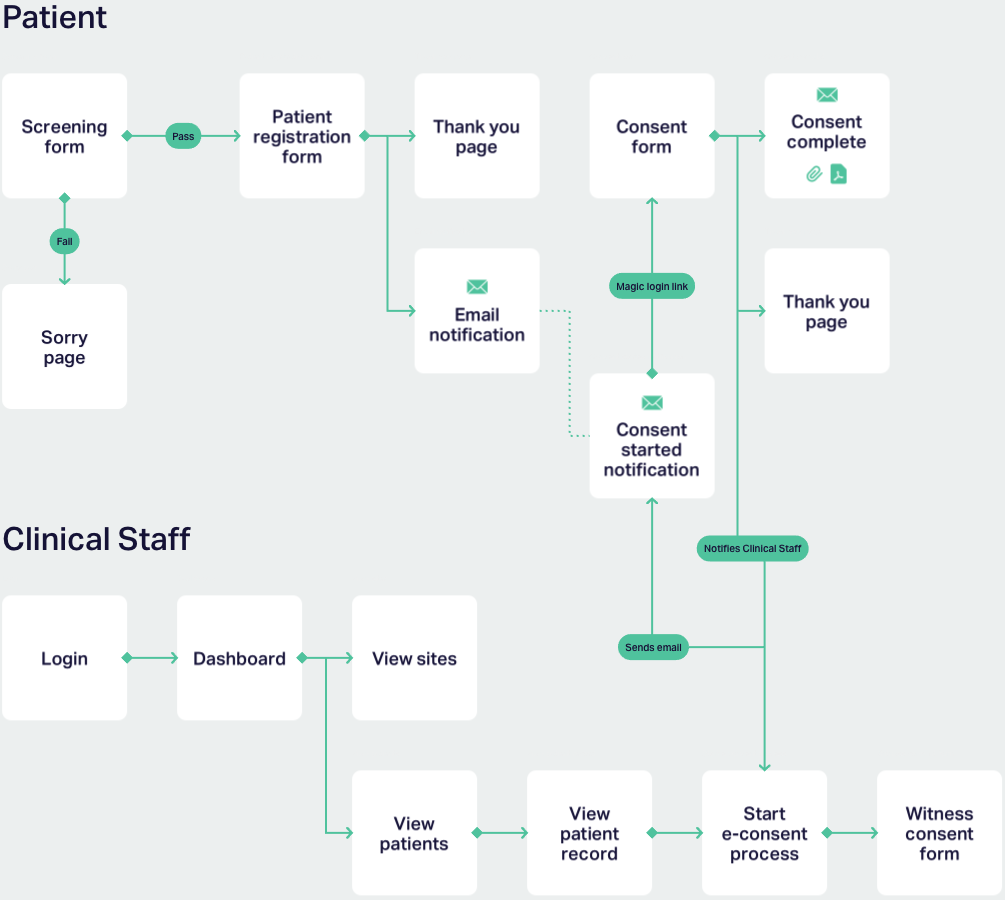
The first problem we solved was designing an electronic consent process that would take a patient-first approach and ensure consent was being accurately provided, whilst being compliant with MHRA standards and an ethics committee review.
What are the right processes to obtain consent?
During our user journey and wireframing sprint we worked with the team to map out the correct patient user journeys in terms of a screening questionnaire, registration form and subsequent consent process. From there, we designed the process for how clinical staff would verify the registration details and witness consent with the patient remotely.
How can we make sure it’s as easy as possible to use for a demographic of older patients?
The trial was targeting an older demographic so we looked for other examples of great accessible design from the Government Digital Service (GDS) and NHS website. Taking a patient-first approach ensure maximum uptake and to provide reassurance of the validity of the trial.
What technology should be used?
We opted for the rapid development framework of Laravel to meet the eight week timescale that was required for the trial to open and maximise patient recruitment whilst the pandemic was just starting. Laravel offers us a rapid design and development process which means we can iterate fast and ultimately provide a more flexible project process to meet our client’s needs.
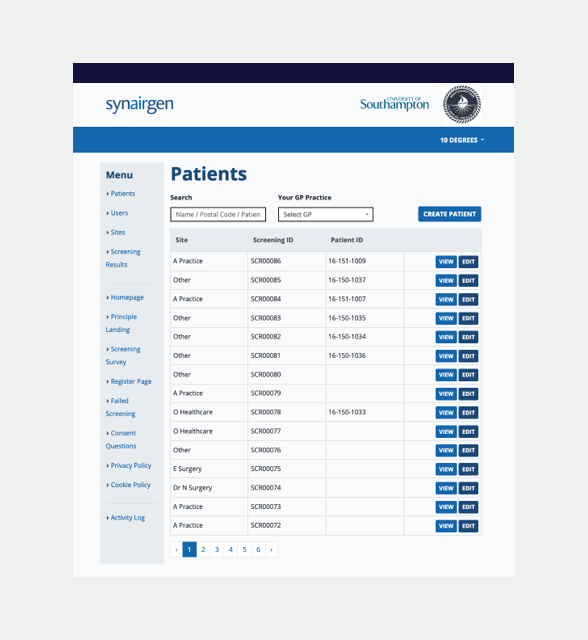
What features are required for management of patient data?
We designed and built the back-end of the product to support a fully compliant content management system with an audit trail that captures all patient data modification. The system generated unique patient IDs to support the randomisation process along with a soft-delete function for data, allowing for full compliance across the clinical trials various data protection policies.
User journey planning sprint:
The 10 Degrees team have done a fantastic job in designing and implementing the SG016 Home trial website to our tight study opening deadline. The level of functionality available has exceeded our expectations and allows us to maintain and manage patient recruitment with ease.”
Jane RobertsonHead of Operations
Southampton Clinical Trial Unit
Our technical solutions
Need a new digital solution that transforms your business? We love the challenge of widely scoped projects and complex integrations.